Misconduct concerns, possible drug risks should stop major stroke trial, whistleblowers say, Science
Por um escritor misterioso
Descrição
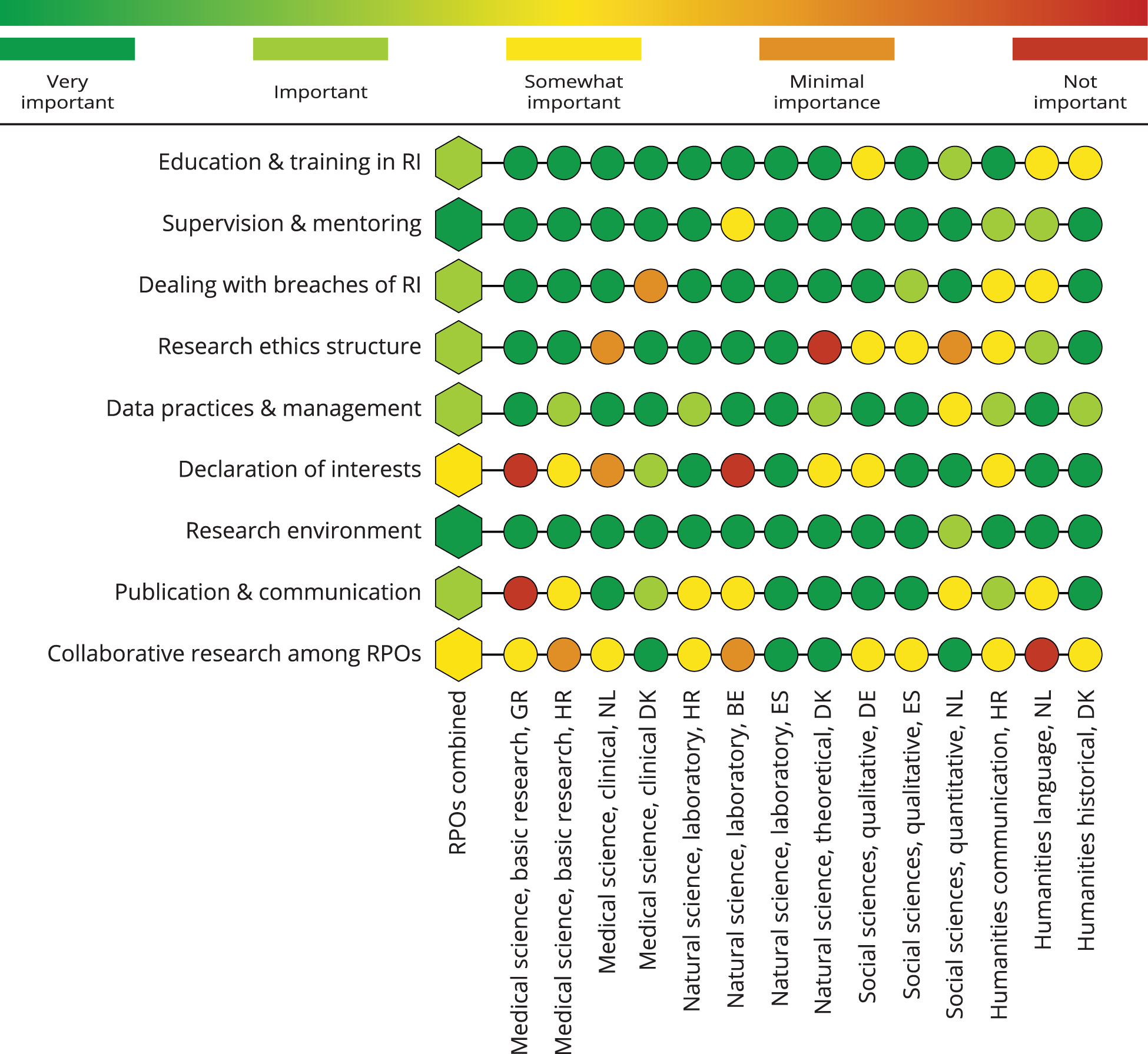
Strengthening research integrity: which topic areas should

NIH puts hold on $30 million trial of potential stroke drug

Corporate Compliance

Full article: Is failure to raise concerns about misconduct a

Russell T. Warne, PhD - Psychologist, author, and educator
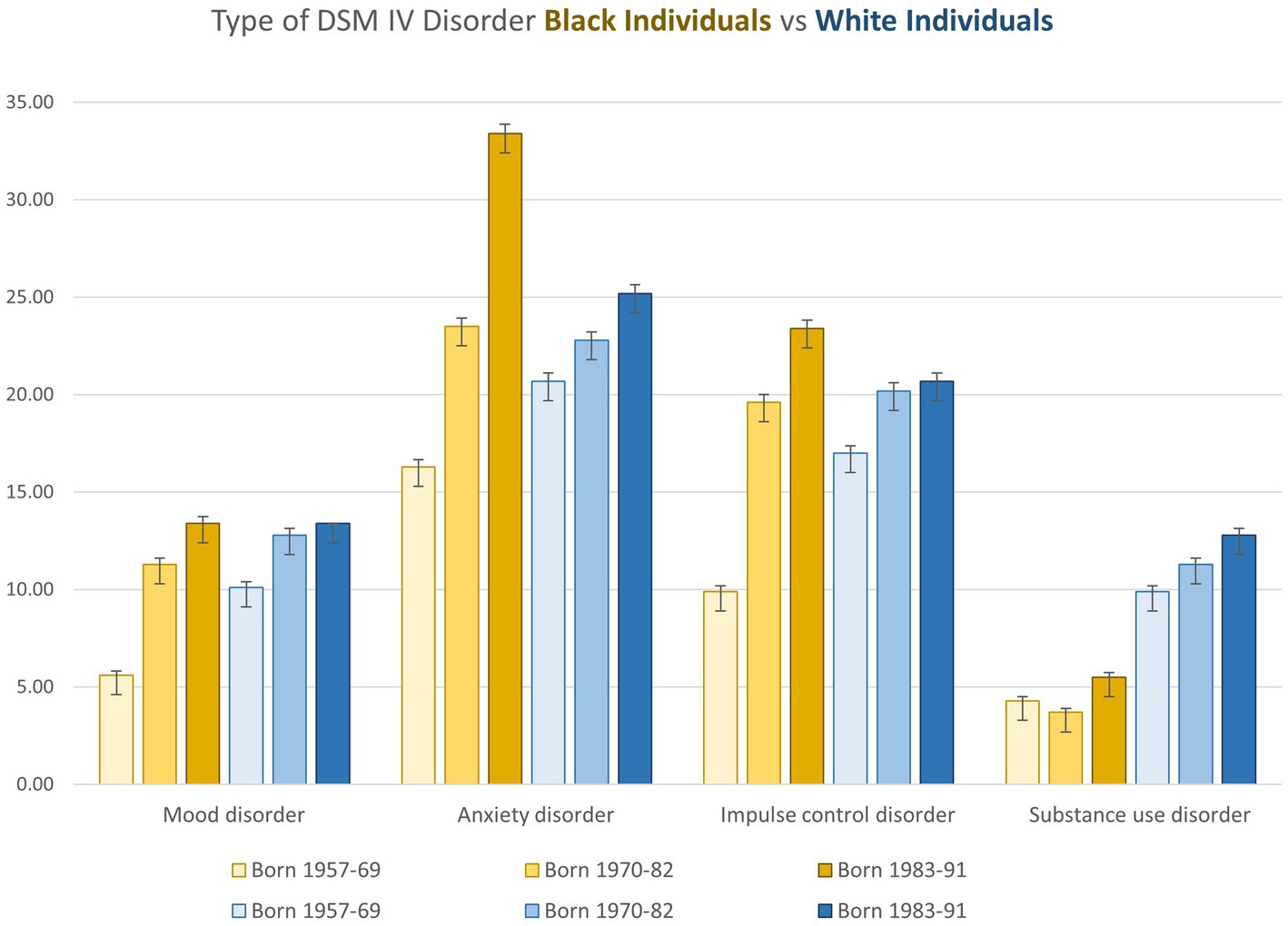
Frontiers The illusion of inclusion: contextual behavioral

Sylvain Lesné is a failed scientist – For Better Science

Life Science Compliance Update November 2016

NIH tells Congress it lacked authority to investigate

Misconduct accounts for the majority of retracted scientific

In all seriousness, what's USC gonna do about its plagiarizing
It's Time to Get Serious About Research Fraud

Bad Pharma: How Drug Companies Mislead by Goldacre, Ben

PDF) How should researchers cope with the ethical demands of
de
por adulto (o preço varia de acordo com o tamanho do grupo)