Early Safety Assessment - Drug Discovery and Development Based on
Por um escritor misterioso
Descrição
The drug candidate faces numerous efficacy and safety hurdles before moving forward to clinical testing. Here at the UPDDI we recognize the need for early identification of potential human toxicity and pharmacokinetic issues by creating a unique human liver microphysiological systems platform for drug testing before implementing preclinical animal testing.

Why 90% of clinical drug development fails and how to improve it

Preclinical Studies

Target safety assessments – identifying risk early

Compound Interest: Understanding the Drug Discovery Process

PDF) Molecular clinical safety intelligence: a system for bridging

Pathology in Nonclinical Drug Safety Assessment - ScienceDirect
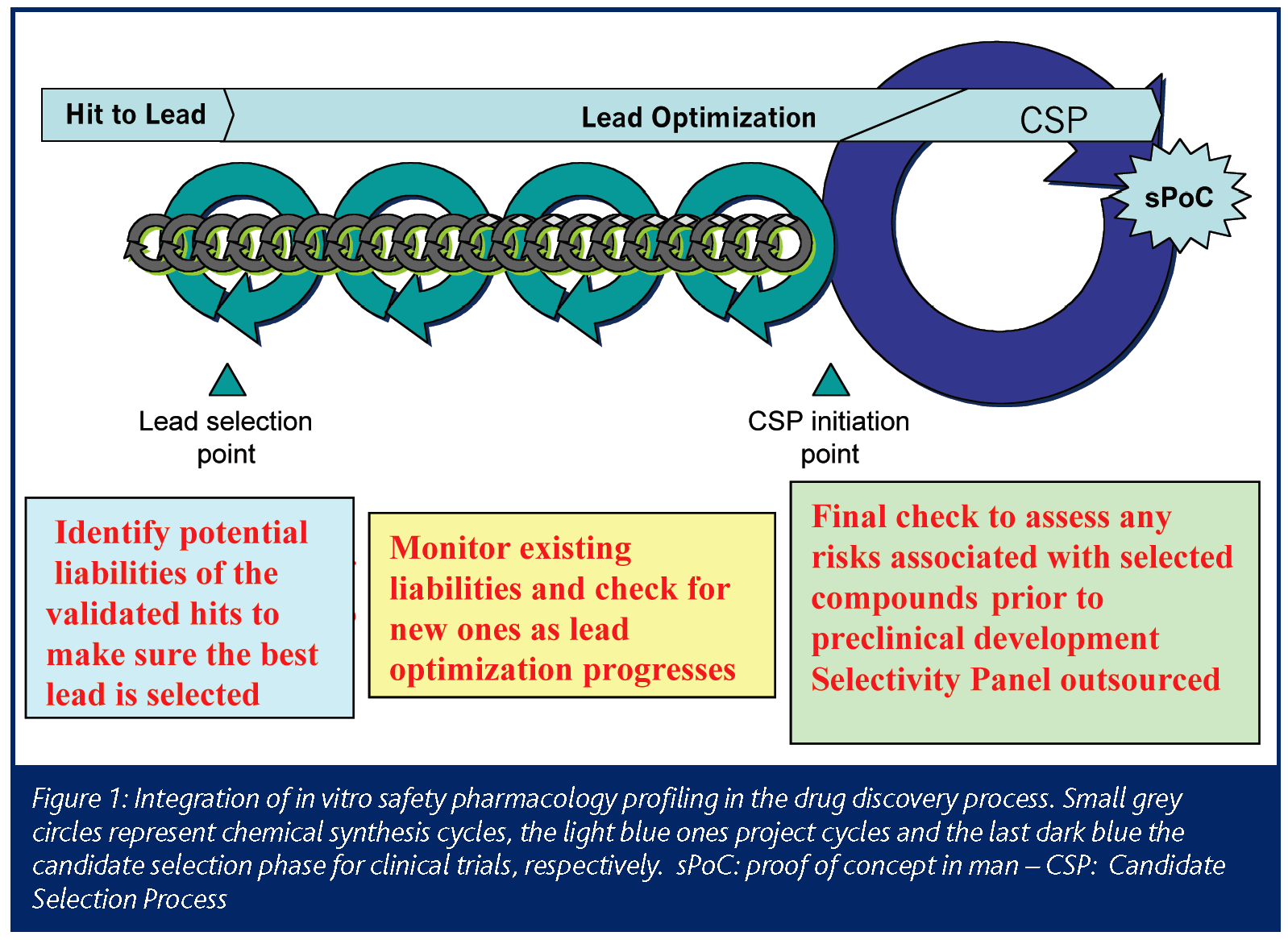
In vitro safety pharmacology profiling - European Pharmaceutical

Drug discovery and development
Target Profiling

Preclinical safety evaluation of biotechnology-derived

How drugs are discovered and developed - The Physiological Society
de
por adulto (o preço varia de acordo com o tamanho do grupo)







